Theoretical discussion on aerosol stability test induced by Arrhenius formula
The necessary process for our aerosol products to be launched is to do stability test, but we will find that although the stability test has passed, there will still be different degrees of corrosion leakage in mass production, or even mass product quality problems. So is it still meaningful for us to do stability test?
We usually talk about 50℃ three months of stability test is equivalent to two years of theoretical test cycle at room temperature, so where does the theoretical value come from? A notable formula needs to be mentioned here: the Arrhenius formula. Arrhenius equation is a chemical term. It is an empirical formula of the relationship between the rate constant of chemical reaction and temperature. A lot of practice shows that this formula is not only applicable to gas reaction, liquid phase reaction and most of the multiphase catalytic reaction.
Formula writing (exponential)
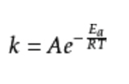
K is the rate constant, R is the molar gas constant, T is the thermodynamic temperature, Ea is the apparent activation energy, and A is the pre-exponential factor (also known as the frequency factor).
It should be noted that Arrhenius' empirical formula assumes that activation energy Ea is regarded as a constant independent of temperature, which is consistent with experimental results within a certain temperature range. However, due to a wide temperature range or complex reactions, LNK and 1/T are not a good straight line. It shows that the activation energy is related to temperature and Arrhenius empirical formula is not applicable to some complex reactions.
Can we still follow Arrhenius' empirical formula in aerosols? Depending on the situation, most of them are followed, with a few exceptions, provided, of course, that the "activation energy Ea" of the aerosol product is a stable constant independent of temperature.
According to Arrhenius equation, its chemical influencing factors include the following aspects:
(1) Pressure: for chemical reactions involving gas, when other conditions remain unchanged (except volume), increase pressure, that is, the volume decreases, the concentration of reactants increases, the number of activated molecules per unit volume increases, the number of effective collisions per unit time increases, and the reaction rate accelerates; Otherwise, it decreases. If the volume is constant, the rate of reaction remains constant at pressure (by adding a gas that does not take part in the chemical reaction). Because the concentration doesn't change, the number of active molecules per volume doesn't change. But at constant volume, if you add the reactants, again, you apply pressure, and you increase the concentration of the reactants, you increase the rate.
(2) Temperature: as long as the temperature is raised, the reactant molecules gain energy, so that part of the original low energy molecules become activated molecules, increasing the percentage of activated molecules, increasing the number of effective collisions, so that the reaction rate increases (the main reason). Of course, due to the increase of temperature, the rate of molecular movement is accelerated, and the number of molecular collisions of reactants per unit time is increased, and the reaction will be accelerated accordingly (secondary cause).
(3) Catalyst: the use of positive catalyst can reduce the energy required for the reaction, so that more reactant molecules become activated molecules, greatly improving the percentage of reactant molecules per unit volume, thus increasing the rate of reactants thousands of times. Negative catalyst is the opposite.
(4) Concentration: When other conditions are the same, increasing the concentration of reactants increases the number of activated molecules per unit volume, thus increasing the effective collision, reaction rate increases, but the percentage of activated molecules is unchanged.
The chemical factors from the above four aspects can well explain our classification of corrosion sites (gas phase corrosion, liquid phase corrosion and interface corrosion) :
1) In gas phase corrosion, although the volume remains unchanged, the pressure increases. As the temperature rises, the activation of air (oxygen), water and propellant increases, and the number of collisions increases, so the gas phase corrosion is intensified. Therefore, the selection of appropriate water-based gas phase rust inhibitor is very critical
2) liquid phase corrosion, due to the activation of increased concentration, some impurities may (such as hydrogen ions, etc.) in a weak link and packaging materials accelerated collision produced corrosion, so the choice of liquid phase antirust agent should be considered carefully combined with pH and raw materials.
3) Interface corrosion, combined with pressure, activation catalysis, air (oxygen), water, propellant, impurities (such as hydrogen ions, etc.) comprehensive reaction, resulting in interface corrosion, the stability and design of the formula system is very key.

Back to the previous question, why is it that sometimes the stability test works, but there is still an anomaly when it comes to mass production? Consider the following:
1: stability design of formula system, such as Ph change, emulsification stability, saturation stability and so on
2: impurities in the raw material exist, such as changes in hydrogen ions and chloride ions
3: batch stability of raw materials, ph between batches of raw materials, content deviation size and so on
4: the stability of aerosol cans and valves and other packaging materials, the stability of the thickness of tin plating layer, the replacement of raw materials caused by the price rise of raw materials
5: Carefully analyze every anomaly in stability test, even if it is a small change, make a reasonable judgment through horizontal comparison, microscopic amplification and other methods (this is the most lacking ability in domestic aerosol industry at present)
Therefore, product quality stability involves all aspects, and it is necessary to have a complete quality system to control the whole supply chain port (including procurement standards, research and development standards, inspection standards, production standards, etc.) to meet the quality standard strategy, so as to ensure the final stability and conformity of our products.
Unfortunately, what we want to share at present is that stability testing cannot guarantee that there are no problems in stability testing, and mass production must have no problems. Combining the above considerations and stability testing of each product, we can prevent the vast majority of hidden dangers. There are still some problems waiting for us to explore, discover and solve. One of the attractions of aerosols is that more people are expected to solve more mysteries.
Post time: Jun-23-2022





